New Xylella fastidiosa outbreak detected in Portugal
About a month ago, the national program for surveys of organisms in quarantine in Portugal reported the presence of the bacterium Xylella fastidiosa (Xf) in 10 new localities in the surroundings of Oporto. In the last few years, the bacterium has been detected a few times in the country, while it has been established in Mallorca and Puglia causing devastating consequences. As stated in La Voz de Galicia regional newspaper, the proximity of Oporto to the Spanish region of Galicia has raised the alarms among the plant health managers of the region. There is a special concern for the possibility of a Pierce’s Disease (PD) outbreak, which affects the vineyards, a relatively important economic sector of the region.
Recently, we developed a temperature-driven dynamic epidemiological model to assess the risk of the establishment of PD worldwide. The model is based on three basic ingredients: the climatic conditions that allow for the survival and reproduction of the bacterium (i.e temperature), the presence of vectors that can spread the disease (i.e. Philaenus spumarius in Europe), and the usual temporal dynamics of disease spreading (i.e. an initial exponential regime given by the usual branching processes). So, what can our model say about this recently detected outbreak?
We ran our model using high-resolution daily temperature data from the Chelsa dataset in the period 2008-2016. We used the same parameters as used in our original paper published in Communications Biology:
-
The Basic Reproductive Number for Europe, \(R_0=5\)
-
The climatic suitability for the main European vector of the disease, Philaenus spumarius, derived from a Species Distribution Model (SDM), as a proxy for its presence and abundance.
Check the paper for a detailed description of the model and all the technical details or check this post for a non-technical summary about it!
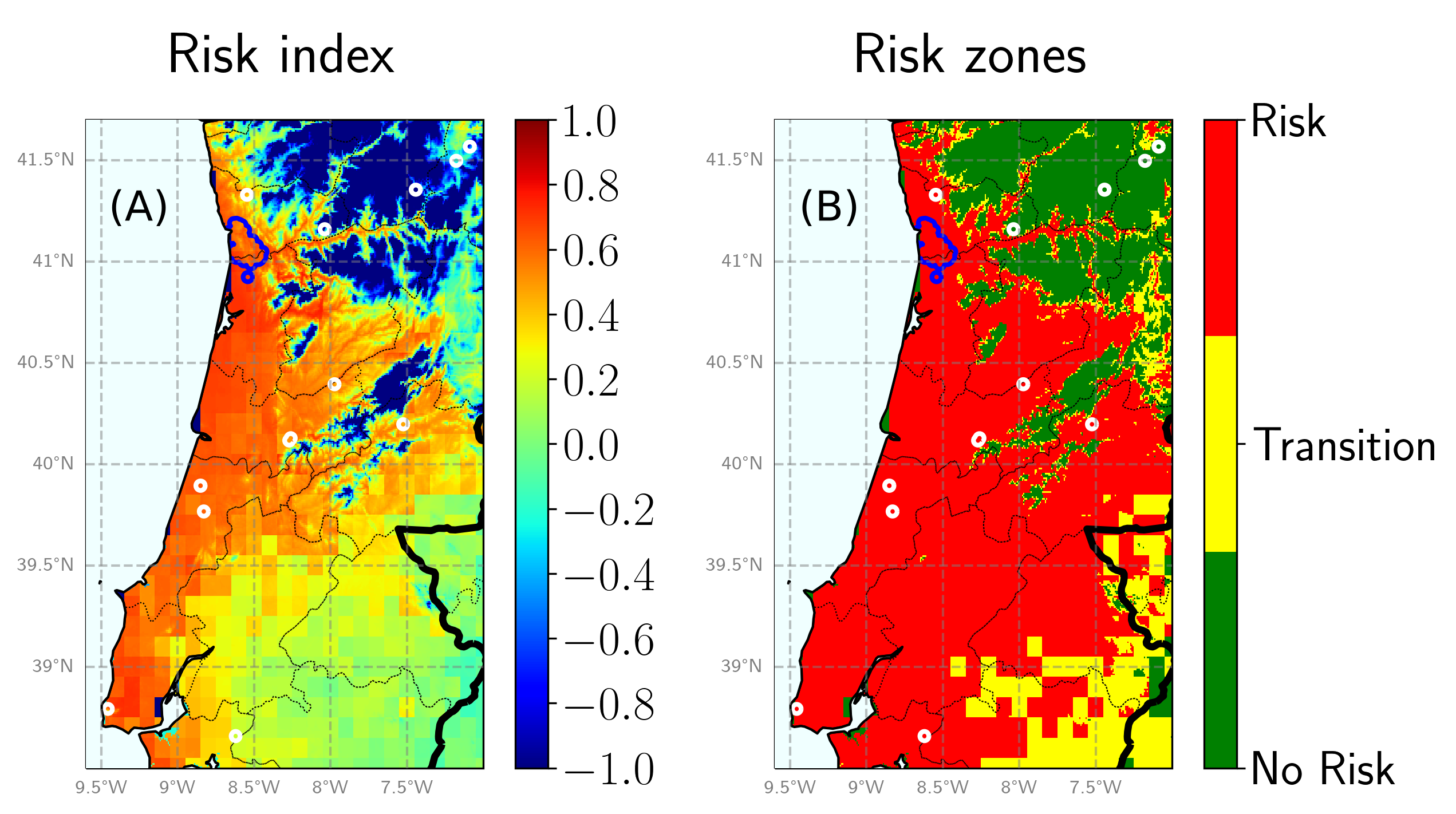
Fig. 1. Risk of PD establishment together with the zones in which Xf has been detected. The blue demarked zone corresponds to the zone of the recently detected outbreak while white circles correspond to previous detections. (A) Disease risk index (i.e. normalized growth rate of simulated incidence). (B) Risk zones.
We observe that almost all zones in which Xf has been detected are inferred as risk zones by the model. Few of them seem to remain in no-risk zones, but we must recall that detections do not imply general propagation and subsequent establishment. Indeed, given that our model is mechanistic (i.e. process-based), we can further analyse our results and try to find why there is a risk or not in the different zones.
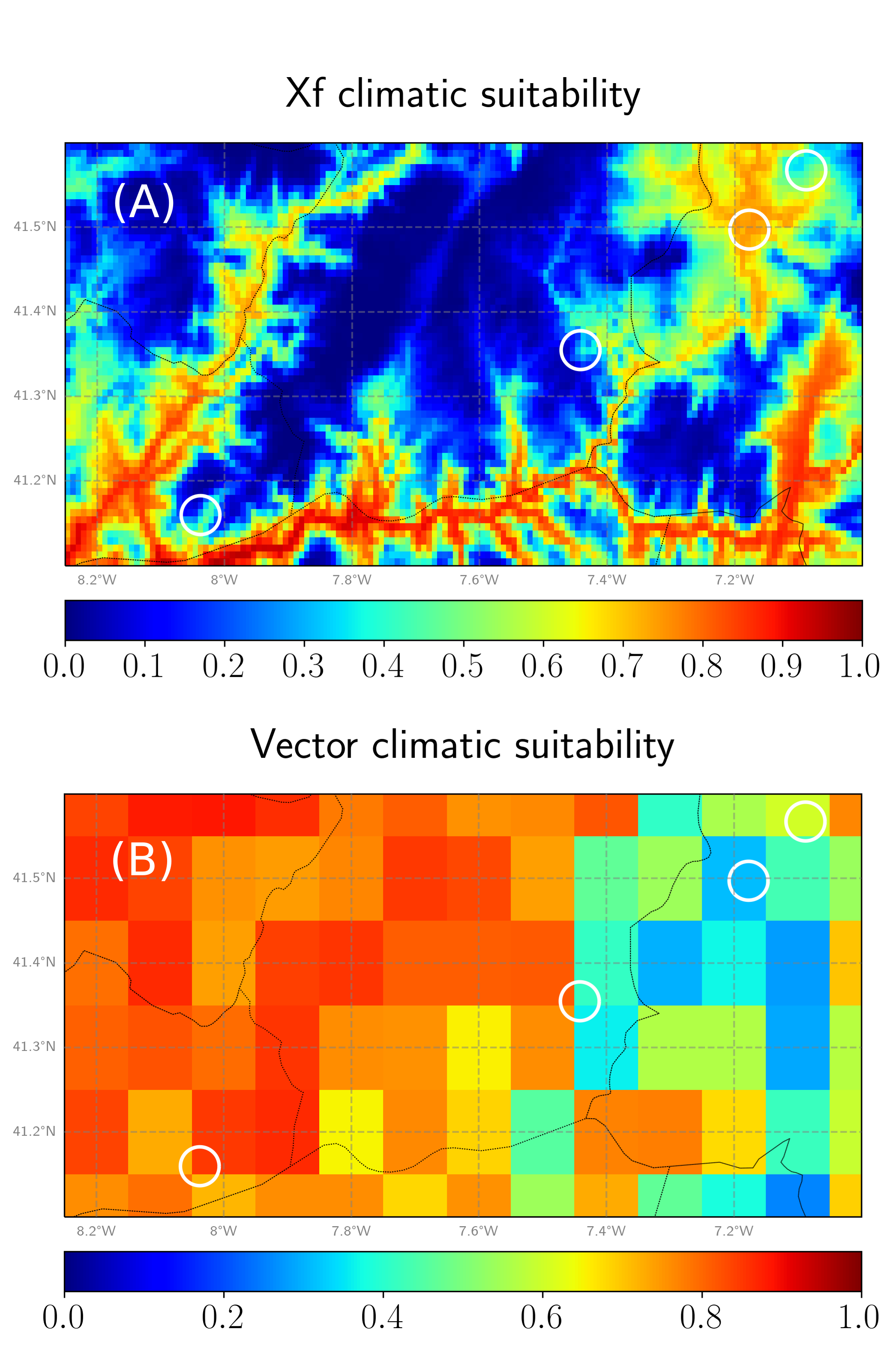
Fig. 2. (A) Climatic suitability of Xf. (B) Climatic suitability for the main European vector, Philaenus spumarius.
In Fig. 2 we show the temperature-based climatic suitability of Xf (Fig. 2A) and the vector climatic suitability (Fig. 2B) around the regions that were inferred as no-risk zones in our model. We observe that most of these zones are indeed climatically suitable for the bacterium, but there is not enough vector presence to propagate it and produce an outbreak. Nevertheless, the vector climatic suitability is only a proxy for vector presence and abundance, and the values come from a correlative model (SDM), so it is not the more robust part of our model. In other words, if vector abundance in this zones was underestimated by the SDM, then it could be a risk zone in practice.
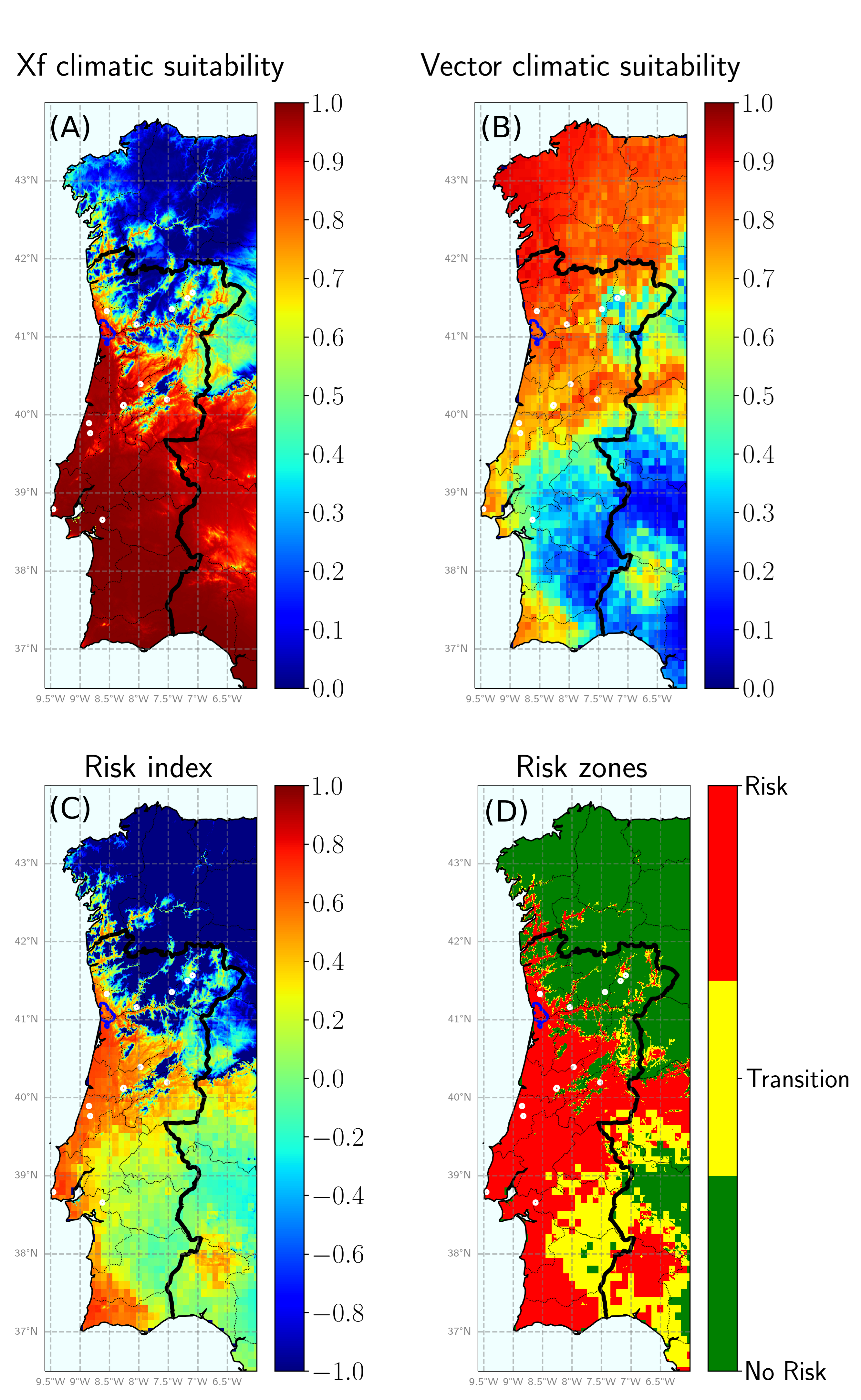
Fig. 3. (A) Climatic suitability of Xf. (B) Climatic suitability for the main European vector, Philaenus spumarius. (C) Risk index. (D) Risk zones. Again, the blue demarked zone corresponds to the zone of the recently detected outbreak while white circles correspond to previous detections.
We can even go further with our model and try to assess the risk of PD establishment in Galicia. In Fig. 3 we show the climatic suitability of Xf (Fig. 3A), the vector climatic suitability (Fig. 3B), the risk index (Fig. 3C) and the risk zones (Fig. 3D) for both Portugal and Galicia. Similarly to the previous reasoning, we can argue that the probability of having a major PD outbreak in Galicia is low. Although the vector is abundant, we observe that the region is not in general climatically suitable for the bacterium. However, note that this is not the case for the surroundings of the Miño river and the western coast, so most of the prevention efforts should be focused on these zones. Furthermore, climate change will for sure alter the climatic suitability of Xf in these zones, making them more suitable, but this is a topic for another day!
I hope to have convinced you not only of the predicting power of our model but also of its high explainability. By now, I think that the latter is one of the most valuable features of mechanistic models that still can not be achieved with correlative ones, i.e. Artificial intelligence.




